Febrile Temperature Critically Controls the Differentiation and Pathogenicity of T Helper 17 Cells
- https://doi.org/10.1016/j.immuni.2020.01.006
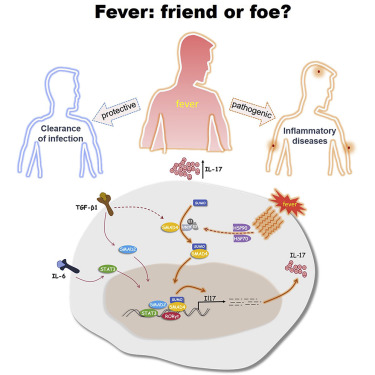
本文要点:
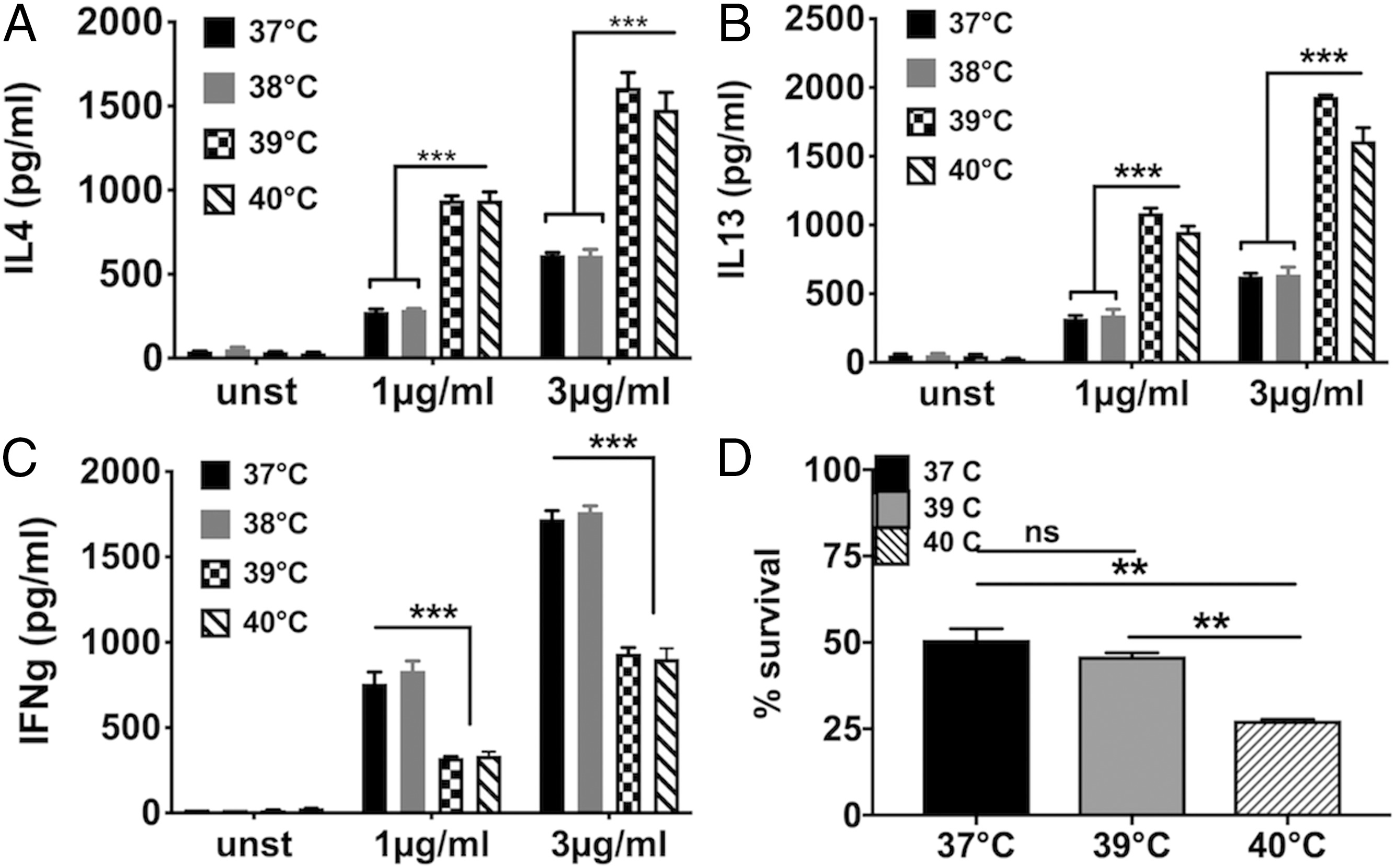
1)与37°C以下相比,在发热温度(38.5°C–39.5°C)下生成的Th17细胞具有强致病性基因的表达,并具有体内促炎活性。体外高温选择性地调节Th17细胞分化以增强白介素17(IL-17)、IL-17F和IL-22表达,该结果证明发烧在适应性免疫反应中起关键作用,并影响了自身免疫性疾病。
2)从机制上讲,发热温度促进了SMAD4转录因子的SUMO化,以促进其核定位。
3)在体外以及改良的自身免疫性疾病模型中,SMAD4的缺失有选择地消除了发热温度对Th17细胞分化的影响
- Febrile temperature change modulates CD4 T cell differentiation via a TRPV channel-regulated Notch-dependent pathway
https://doi.org/10.1073/pnas.1922683117
Since fever is frequently a response to infection, immune responses likely occur commonly at fever temperatures. Are fever temperatures detected by immune cells, what molecular pathways are involved in this sensing, and what are the functional consequences? Our data show that CD4 T lymphocytes detect moderate fever temperature. A TRPV channel-mediated pathway involving Notch activation leads to a Th2 skewing of the effector T cell response. However, the antigen presenting dendritic cells that stimulate CD4 T cells, in turn, detect fever temperature and overproduce interleukin-12 to counter this Th2 skewing. The evolutionary genesis of these pathways and the functional consequences of this complex regulatory loop in specific infectious circumstances are, thus, likely to be of great interest.
- Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism
In summary, we have shown that increased transamination via GOT1 leads to a much greater accumulation of 2-HG in differentiating TH17 cells than in iTreg cells, and this promoted the methylation of the Foxp3 gene locus and silenced Foxp3 gene expression. Notably, we found that 2-HG levels in differentiating TH17 cells (for example, 0.1–0.4 mM) were much lower than that in human cancer cells containing IDH1 and IDH2 mutations (≥1 mM). Nonetheless, endogenous 2-HG accumulations under TH17 conditions and experiments with exogenously added 2-HG in TH17 cell culture correlate well with hypermethylation of the Foxp3 gene locus and reduced mRNA and protein levels of FOXP3 in fully differentiated TH17 cells, suggesting that different cell types may exhibit differential sensitivity to 2-HG levels. Manipulating a single step in a glutamate metabolic pathway could change TH17 cell fate by affecting methylation of the Foxp3 gene locus, and ameliorate mouse EAE disease by regulating the TH17 and iTreg cell balance, highlighting the importance of cellular metabolism in the determination of T-cell fate.