CAR T-cell Therapy
Cancer therapy — There are several approaches to using gene therapy as a component of cancer treatment. Some of these may be appropriate for hematologic malignancies as well as solid tumors. Unlike correction of single gene disorders, gene therapy for cancer that attempts to kill malignant cells presents additional challenges related to additional mutations or immunologic changes used by the cancer cells to survive.
Examples include the following:
●Antitumor immune response – Gene therapy can be used to augment the immune response and immune killing of tumor cells. As examples:
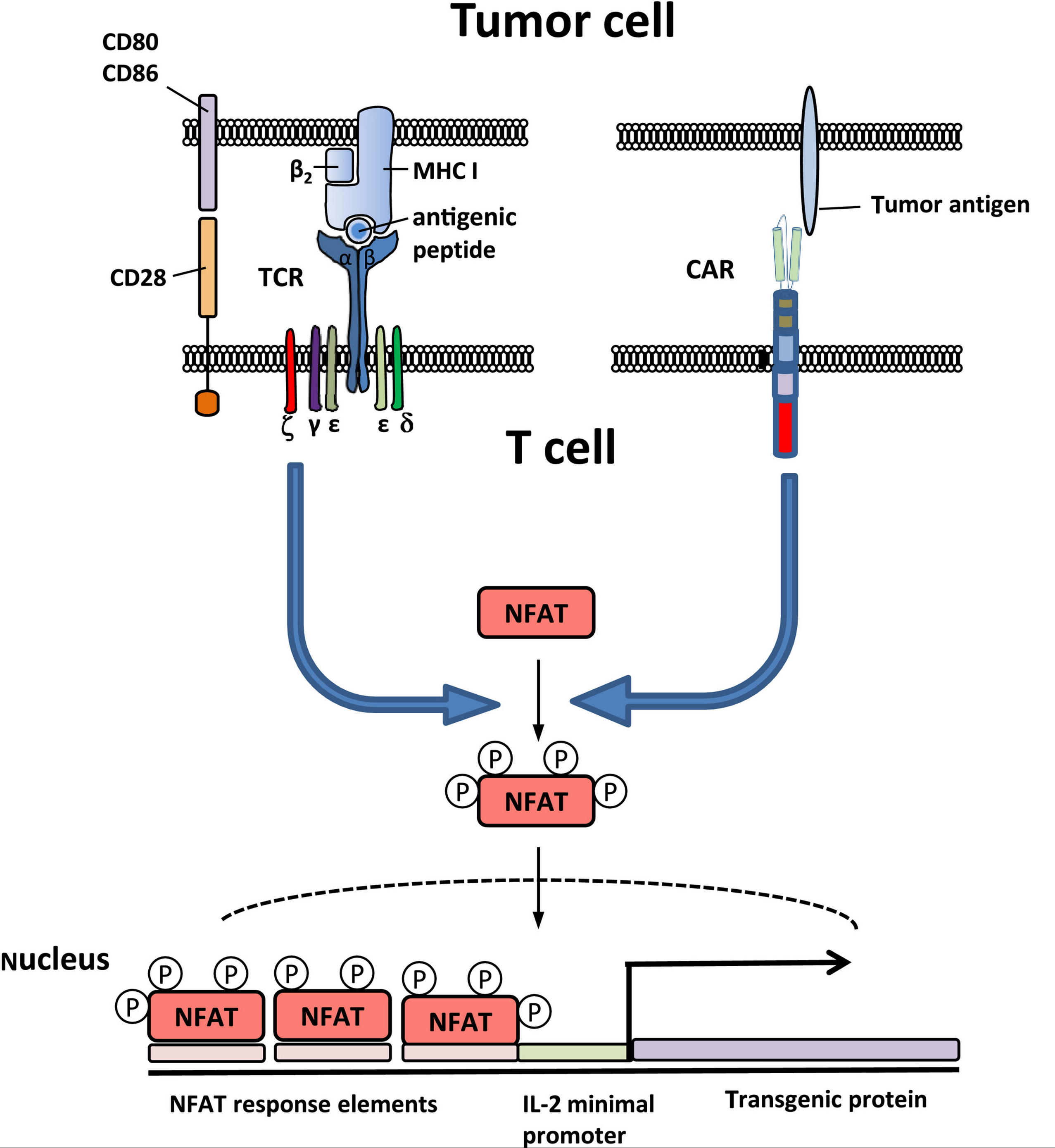
•Ex vivo modification of autologous T lymphocytes to make them express a chimeric antigen receptor (CAR) can be used to direct the patient's own lymphocytes to their own tumor cells; this is referred to as CAR-T therapy or T-cell immunotherapy. The use of T cells for CAR therapy has shown remarkable efficacy in certain lymphoid malignancies . Commercial CAR-T cell products (eg, tisagenlecleucel, axicabtagene ciloleucel) became available for clinical use in 2017. The use of HSCs for CAR therapy is under investigation . Clinical use of CAR-T therapies is presented separately. (See "Treatment of relapsed or refractory acute lymphoblastic leukemia in adults", section on 'CAR-T' and "Multiple myeloma: Treatment of third or later relapse", section on 'Chimeric antigen receptor T cells' and "Diffuse large B cell lymphoma (DLBCL): Suspected first relapse or refractory disease in medically-fit patients", section on 'Anti-CD19 CAR-T cell therapy' and "Diffuse large B cell lymphoma (DLBCL): Second or later relapse or patients who are medically-unfit", section on 'CAR-T cell therapy'.)
•Talimogene laherparepvec (T-VEC, Imlygic) is a modified herpes simplex virus (HSV) engineered to express granulocyte-macrophage colony-stimulating factor (GM-CSF); it was approved in 2015 in the United States and Europe for treating unresectable melanoma. The virus is injected directly into cutaneous, subcutaneous, or nodal lesions; the dose is based on the size of the lesion. Its mechanism is thought to involve recruitment and activation of immune cells to the site of the tumor. In a trial in which 436 patients were randomly assigned to receive T-VEC or subcutaneous GM-CSF, T-VEC was associated with higher rates of overall response and durable response and a trend towards better overall survival (hazard ratio [HR] 0.79; 95% CI 0.62-1.00) . This therapy and its use in melanoma are discussed in more detail separately. (See "Cutaneous melanoma: Management of local recurrence".)
Other gene therapies targeting the immune response are under investigation.
•Modification of other immune cells such as natural killer (NK) cells or macrophages to express a CAR is also possible. A proposed advantage of this approach is that autologous cells are not required to generate the CAR cells, which means the therapy could be used as an allogeneic "off the shelf" product; clinical trials with such products suggest that these approaches are effective. Even if the host mounted an immune response against the CAR-NK or CAR-macrophages, it might take several days, during which the CAR cells could continue killing the tumor. (See "Treatment of relapsed or refractory chronic lymphocytic leukemia", section on 'Chimeric antigen receptor T cells'.)