Abscopal effect

1953年,Mole等发现照射局部组织可以在远离照射野的相同或不同组织中引发生物响应,并提出“异位 (abscopal)”的概念。人们在转移瘤的临床治疗中发现,对一个特定的肿瘤部位进行局部照射,能够引发非照射野的肿瘤体积出现明显缩小甚至消失,这个现象被称为远端效应 (abscopal effect)。
R.H. Mole proposed the term “abscopal” (‘ab’ - away from, ‘scopus’ - target) in 1953 to refer to effects of ionizing radiation “at a distance from the irradiated volume but within the same organism.”
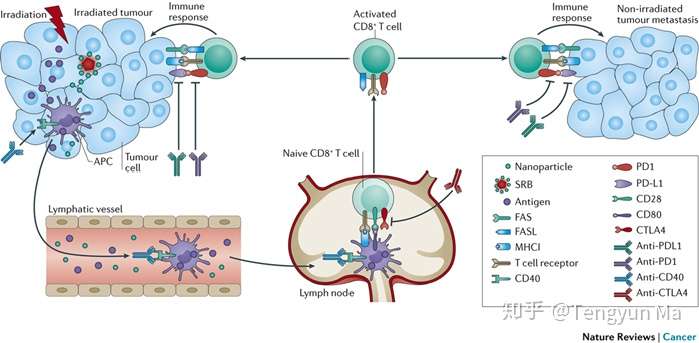 图:Radiation generates neoantigens from tumour cells. Antigens from damaged tumour cells can be taken up by antigen-presenting cells (APCs), which travel to the lymph node to prime the T cell-mediated abscopal effect. Activated T cells directed against tumour-specific antigens t
图:Radiation generates neoantigens from tumour cells. Antigens from damaged tumour cells can be taken up by antigen-presenting cells (APCs), which travel to the lymph node to prime the T cell-mediated abscopal effect. Activated T cells directed against tumour-specific antigens t
目前认为放射治疗诱导远隔效应可能的免疫学机理主要有以下两点:
1.放射治疗的原位疫苗效应:有学者认为远隔效应的产生可能与放疗诱发产生原位疫苗有关。局部放疗引起肿瘤细胞死亡,通过“免疫原性细胞死亡”释放免疫源性因子,随后触发一批内源性损伤相关的分子模型分子(damage associated molecular patterns,DAMPs)的释放,包括:钙网蛋白,高迁移率组蛋白B1、ATP。他们触发树突状细胞(dendritic cells,DC),增加抗原提呈给T细胞。
2.放射治疗重构肿瘤免疫微环境:研究发现,远隔效应还与放疗重构肿瘤微环境有关。放疗可使肿瘤微环境有益于效应T细胞的募集和功能作用。可以诱使趋化因子参与效应T细胞的补充,有效地使肿瘤变为“炎性”组织,而易被T细胞攻击。放疗可能对肿瘤微环境中的基质细胞和肿瘤细胞本身产生直接的促炎作用,从而促进建立一个可接受的免疫介导的肿瘤排斥的微环境。
*Abscopal response mechanism.* Although the biological mechanism underlying the abscopal effect is yet to be fully understood, several studies have helped to elucidate how combining radiotherapy with immunotherapy would boost this effect. When a tumour is irradiated, the cellular stress or injury in the tumours may lead to the liberation of neoantigens, here referred to as tumour-associated antigens (TAAs), in the context of necrotic and apoptotic tumour cells and debris. A substantial increase in the number and diversity of TAAs can stimulate a tumour-specific immune response, with TAAs engulfed by antigen-presenting cells (APCs) and then presented to CD8+ T cells. The CD8+ T cells can then recognize and attack both the primary tumour and metastatic disease28 (Fig. 2). Irradiated tumour cells may also release cellular danger-associated molecular patterns (DAMPs) and cytokines that enhance traffic of immune cells29. Collectively, these events promote tumour cell elimination by primed CD8+ T cells
The rarity of the abscopal effect suggests that even primed antitumour CD8+ T cells are unable to overcome the suppressive effect of the tumour microenvironment29,30. Immunosuppressive cytokines released by tumours, such as transforming growth factor-β (TGFβ), and surface receptors expressed on T cells, such as CTLA4, can inhibit the function of T cells. M2 macrophages, myeloid-derived suppressor cells (MDSCs) and immature dendritic cells (DCs) may also suppress T cell functions29,30. Tumour elimination could be further inhibited by CD4+ T cells with regulatory function (also called regulatory T (Treg) cells).
For a range of tumour types, preclinical studies (Table 1) consistently show that combining radiotherapy with immunotherapy substantially improves abscopal response rates compared with using radiotherapy or immunotherapy alone. From these studies, it is also evident that different immunotherapeutic agents can boost abscopal responses by targeting different aspects of the immune-mediated response9,17,27. For example, FLT3L can be used to recruit and stimulate APCs17,18,32, anti-CD40 can be employed to increase activation of APCs33, and anti-CTLA4 or antibodies against programmed cell death protein 1 (PD1) can act as immune checkpoint inhibitors, increasing the T cell activity directed against tumour cells
Proposed mechanism of the abscopal effect, mediated by the immune system. Here, local radiation causes tumor cell death, which is followed by adaptive immune system recognition, not unlike a vaccine.
Ngwa, W., Irabor, O., Schoenfeld, J. et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer18, 313–322 (2018). https://doi.org/10.1038/nrc.201